Restriction Enzymes: A History
By Wil A.M. Loenen, Leiden University Medical Center
April 2019 · 346 pages, illustrated (38 color and 26 B&W)
ISBN 978-1-621821-05-2
<< Introduction — Chapter 2 >>
Chapter 1
Chapter doi:10.1101/restrictionenzymes_1
Discovery of a Barrier to Infection and Host-Controlled Variation: 1952–1953
While I was focusing on P2 and the mechanism of lysogeny, some unexpected findings came up which deserved proper attention. One was the discovery of “host controlled variation,” now more commonly called “restriction and modification,” a phenomenon of great theoretical interest. I noticed it in P2 (using strain B as the restricting host, Shigella being the standard host) and did not know what to make of it. Jean Weigle noticed it in lambda (using strain C as the permissive host, K-12 being the standard host): being aware of my results, he immediately recognized the parallelism of the two “systems.”
Thus Giuseppe (Joe) Bertani recalls events leading up to his joint publication with Jean Weigle of “Host controlled variation in bacterial viruses” (Bertani and Weigle 1953) in a letter to Noreen Murray (July 17, 2003).1
Joe Bertani obtained his doctor's degree in zoology at the University of Milan soon after World War II. Via Zürich, Naples, and Cold Spring Harbor, he started working on lysogeny in Bloomington in 1951 with Salvador Luria, a man of “brilliance, integrity, breadth of culture, and wicked sense of humor,” according to Evelyn Witkin. Here Joe Bertani shared a bench for a while with James Watson, of later double helix fame.
Lysogeny is the ability of some viruses to be carried in a dormant “prophage” state in their bacterial host chromosome. The discovery by Esther Lederberg of prophage lambda in Escherichia coli K12, and that of P1 and P2 by Joe Bertani, opened research into the different aspects of genetic exchange in phage and host bacteria in the same genetic background. These phages would prove useful tools in molecular biology: P1 encodes its own restriction-modification (R-M) system, EcoP1I, later to be classified as Type III; it can package and transfer foreign DNA allowing genetic exchange with a new host (generalized transduction). This led to the development of the highly useful LoxP-Cre recombination system, a topic outside the scope of this book (see, e.g., Yu and Bradley 2001). Phage P2 would prove useful in cloning schemes in the 1970s and 1980s because of the “spi− phenotype” (susceptibility to P2 inhibition): P2 lysogens exclude growth of wild-type lambda (see, e.g., Hershey 1971, p. 146), allowing selection for recombinant phage in the generation of libraries in lambda gene bank vectors. In 2001, Joe Bertani was honored at the Molecular Genetics of Bacteria and Phages meeting (Fig. 1) that marked the 50th anniversary of the discovery of the three classic phages: lambda, P1, and P2 (Young 2002). The importance of lambda needs no further explanation (Hershey 1971).

FIGURE 1. Joe Bertani and two other pioneers of phage and bacterial genetics (2001); from left to right: Abe Eisenstark, Joe Bertani, and Wacław Szybalski (taken at the meeting in Madison, Wisconsin). (Reprinted from Young 2002, with permission from the American Society for Microbiology.)
Jean Weigle started his career at the University of Geneva in physics. After a heart attack he quit his professorship and joined Max Delbrück at the California Institute of Technology (Caltech), working on transduction and recombination until his death. He continued to spend summers at the Kellenberger laboratory in Geneva, which led Werner Arber to a postdoctoral year with Joe Bertani. Thus, Werner Arber recognized host-controlled variation (HCV) in his own experiments 7 years later. Renamed restriction and modification, he would be awarded the Nobel Prize in 1978, together with Hamilton (Ham) Smith and Daniel Nathans.
In his letter, Bertani recalls his interest in the mechanism of P2 lysogeny that led him to encounter the phenomenon of R-M. P2 was usually grown on Shigella, but this phage only rarely gave plaques on E. coli B, from which background P2 had been isolated. What about the opposite effect? Grow P2 on E. coli B and passage the outcoming phage on Shigella. In November 1951, Bertani did his crucial “one-step growth” experiment and cycled P2 on the two strains. The results were clear: Progeny from E. coli B (P2·B) plated with 100% efficiency (e.o.p.=1) on E. coli B and Shigella, but phage grown on Shigella (P2·Sh) only plated with 0.01% efficiency on E. coli B (e.o.p.=10−4), compared to the titer on Shigella.
By this time, Joe Bertani was on good terms with Jean Weigle at Caltech, who noted a similar pattern cycling lambda on different E. coli strains. Lambda grown on E. coli C (lambda·C) grew with an e.o.p. of ∼2×10−4 on a E. coli K12 derivative that had been cured of prophage lambda (called K12S; S in Fig. 2). Having passed this barrier to productive infection, the surviving phages were now fully capable again of growth on E. coli K12 but were “restricted” by E. coli B (lambda·B). Their preliminary results were published independently in the Microbial Genetics Bulletin (MGB6) in April 1952, a full year before the publication of the DNA helix structure. Both realized that this was probably a very general phenomenon. They published their unexpected results in a joint paper, although no satisfactory mechanistic explanation was in sight at the time (Bertani and Weigle 1953). At the end of their paper they present a picture to show the parallel between the barriers to lambda and P2 (Fig. 2).
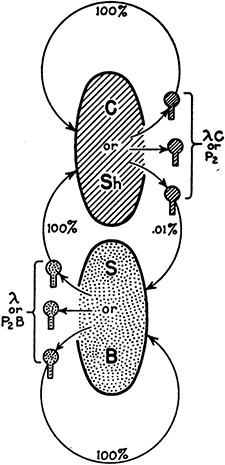
FIGURE 2. Homologies between the phage P2 and λ systems of host range variation. Lines and stipples in the phages represent phage structures whose specificity is completely or almost completely determined by the host cells in which the phage was produced and which are similarly lined or stippled. The percentages indicate the efficiency of plating of a phage on the type of host indicated by the arrow. (Reprinted from Bertani and Weigle 1953, with permission from the American Society for Microbiology.)
It was clear from their experiments that lambda·K was not a genetic mutant of lambda·C, and it was concluded that the modifying property was “host-controlled.” Apparently, E. coli C lacked such a barrier, allowing both lambda·K and lambda·C to grow with an e.o.p. of 1.0. The interpretation proposed assumed “the existence of a phage structure, the specificity of which is completely or almost completely under control of the host cell, and which is required for some step in the process of phage multiplication.”
Around the same time, Salvador Luria and Mary Human published their paper on a barrier to infection by certain T phages (Luria and Human 1952). Their finding of T* phage active on Shigella and not on E. coli B/4o cells that produced it was also very striking. Later it would become clear that T* phage could no longer glycosylate its DNA, thus becoming sensitive to restriction by the mcr system present in E. coli B but absent in Shigella (later designated as a Type IV REase).
About their finding of phage growth on Shigella, but not on the cells that produced it, Joe Bertani wrote: “I don't seem to have thought of it at the time as more than a curiosity to be further investigated, and probably the same applies to some extent to Luria, in so far prior examples of transient phenotype changes were known…. Of course all this was before we had any idea of DNA structure and before we had fully digested the implications of the Hershey & Chase experiment. Besides, there was no certainty at the time that P2 and lambda would resemble the T phages in composition.” It only slowly dawned upon him and Jean Weigle that they faced a breakdown of the distinct picture of genotype and phenotype that had been built up with great care during the first half of the century.
In 1953, Luria summarized all known examples of restriction and modification, called at the time “host-controlled variation,” or “host-induced modifications of viruses” (Luria 1953). He combined and generalized the results of his own phage work, those of Bertani and Weigle, and other data on E. coli, Salmonella, and Staphylococcus phages (Table 1). He concluded that “its [host-controlled variation] outstanding characteristic is that it is strictly phenotypic, nonhereditary, and determined by the host cell, in which the virus has been produced.” Furthermore, these modifications by successive hosts were not accumulative but mutually exclusive. His general scheme would apply to phages P2, lambda, T1, and P1. Evidence for the involvement of DNA in this phenomenon had to await experiments in the early 1960s.
|
TABLE 1. General scheme of adaptive host-induced modification |
|||
|
Efficiency of plating on host |
|||
Phage |
A |
B |
C |
Phage·A |
1 |
10−4 |
10−6 |
Phage·B |
1 |
1 |
10−6 |
Phage·C |
1 |
10−4 |
1 |
Phage·B,C (= P·C) |
1 |
10−4 |
1 |
Phage·C,B (= P.B) |
1 |
1 |
10−6 |
Phage·B,A (= P·A) |
1 |
10−4 |
10−6 |
Phage·C,A (= P·A) |
1 |
10−4 |
10−6 |
|
Adapted, with permission, from Luria 1953, © Cold Spring Harbor Laboratory Press. This scheme would apply to phages P2, lambda, T1, and P1. Strain A is a nonrestricting host, allowing all phages to infect productively. Strains B and C have different restriction systems, and strain C poses a more effective barrier than strain B. (Phage·A) Phage grown on strain A, etc., (Phage B,C) phage grown on strain B, then C; phage will have strain specificity of C, etc. |
|||
Joe Bertani continued his research on lysogeny (Bertani 2004), and silence would reign on the topic of HCV until chance brought Werner Arber into the field 7 years later.
Some suggestions for further reading on these early days are Judson (1979), Luria (1984), Gribbin (1985), Fischer and Lipson (1988), and Lily (1993).
REFERENCES
Bertani G, ed. 2004. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J Bacteriol 186: 595–600. 10.1128/JB.186.3.595-600.2004
Bertani G, Weigle JJ. 1953. Host controlled variation in bacterial viruses. J Bacteriol 65: 113–121.
Fischer EP, Lipson C. 1988. Thinking about science: Max Delbruck and the origins of molecular biology. Norton, New York.
Gribbin J. 1985. In search of the double helix. Quantum physics and life. McGraw-Hill, New York.
Hershey AD. ed. 1971. The bacteriophage lambda. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
Judson HF. 1979. The eighth day of creation. The makers of the revolution in biology, a Touchstone book. Simon and Schuster, New York.
Lily EK. 1993. The molecular vision of life. Caltech, the Rockefeller Foundation and the rise of the new biology. Oxford University Press, New York.
Luria SE. 1953. Host-induced modifications of viruses. Cold Spring Harb Symp Quant Biol 18: 237–244. 10.1101/SQB.1953.018.01.034
Luria SE. 1984. A slot machine, a broken test tube: an autobiography. Harper & Row, New York.
Luria SE, Human ML. 1952. A nonhereditary, host-induced variation of bacterial viruses. J Bacteriol 64: 557–569.
Young R. 2002. Molecular genetics of bacteria and phages, 2001. J Bacteriol 184: 2572–2575. 10.1128/JB.184.10.2572-2575.2002
Yu Y, Bradley A. 2001. Engineering chromosomal rearrangements in mice. Nat Rev Genet 2: 780–790. 10.1038/35093564
APPENDIX 1: LETTER FROM JOE BERTANI TO NOREEN MURRAY, 2003
G. Bertani
Biology 156-29
Caltech
Pasadena, CA 91125
July 17, 2003
Dear Noreen,
More than a month ago I promised to write you within a week or two… My apologies!
The brief paper on my (and others’) old work on lysogeny is not ready yet. Nevertheless, I am copying below what I'll say in it concerning restriction and modification, and follow with some comments.
“…While I was focusing on P2 and the mechanism of lysogeny, some unexpected findings came up which deserved proper attention. One was the discovery of “host controlled variation”, now more commonly called “restriction and modification”, a phenomenon of great theoretical interest. I noticed it in P2 (using strain B as the restricting host, Shigella being the standard host) and did not know what to make of it. Jean Weigle noticed it in lambda (using strain C as the permissive host, K-12 being the standard host): being aware of my results, he immediately recognized the parallelism of the two “systems”. Shortly before that, a minor laboratory accident, as told by Luria (REF A), had led to the discovery of another, albeit more complex case of host controlled variation (REF B). Although no satisfactory mechanistic explanation was in sight at the time, Jean and I were encouraged by the parallelism between our two, totally independent “systems” and decided to publish together our findings (REF C). It rarely happens that a new phenomenon, observed in two different materials, in different labs, is described in the same paper, in a comparative manner. Of course, this strengthened the evidence, hinting at the generality of the phenomenon. It also scored a point for cooperation vs. competition in science and human affairs. A similar case, several years later, was that of a paper by René Thomas and Elizabeth Bertani (REF D), which reported parallel experiments with lambda and with P2 to more precisely define the mode of action of the immunity repressor.”
Ref B=Luria & Human 1952 J.Bact. 64:557
Ref C=Bertani & Weigle 1953 J.Bact. 65:113
Ref D=Thomas & Bertani, L.E. 1964 Virology 24:241
REF A is Luria's autobiography (“A slot machine, a broken test tube”, 1984). He describes the episode that clarified his problem with strains B/4o and B/4oo. I remember the episode a bit differently, but the essential facts are the same. This must have happened in late 1950 or early 1951, since Mary Human, who was doing the experiments, left our lab at the end of March 1951, and I remember having to convince her that using Shigella was not so risky. The finding of phage active on Shigella and not on the B/4o cells that produced it was very striking, but I don't seem to have thought of it at the time as more than a curiosity to be further investigated, and probably the same applies to some extent to Luria, in so far prior examples of transient phenotype changes were known. This is also the impression one gathers from reading the first description of the effect in Luria's abstract for the phage meeting at Cold Spring Harbor, August 20–22, 1951 (in PIS #6 / copy enclosed).
I was mostly interested in lysogeny and had isolated some P2 plaque type mutants to be used as markers in a variety of experiments. Having seen that P2 (which I usually grew on Shigella at that time) only rarely gave plaques on coli B, I presumed these were “host range mutants”. Trying now to reconstruct from my lab notes, it seems that at first I did not see a clear cut effect of the passage from B to Shigella, probably because I was making mostly plate stocks and I was using relatively large phage inocula. I had however some suspicion because I also made, beginning in April 1951, a number of “single clone” experiments, to see the distribution of plaques formed by P2 (grown on Shigella) on coli B: there was no evidence of a clonal distribution. I don't know at which point I started worrying about the loss of the ability to plate on B by P2 grown on B and then passaged on Shigella: my first “neat” one-step growth experiment showing this is of November 1951.
I have not succeeded in reconstructing when I first met Jean Weigle, whether in 1950 or in 1951, but by the Fall of 1951 we were in very good terms, corresponding by letter and exchanging strains. He knew about my problems concerning P2 and coli B vs. Shigella. In late 1951 he wrote me a letter giving data on efficiencies of plating of lambda on various indicator strains, and showing that the “lambda/K-12/122” (122 being what we later called strain C) pattern of plating could perfectly parallel the “P2/B/Sh” pattern, except that we were starting from opposite ends. That is when (I believe) we all realized that this was probably a very general phenomenon. I proposed to Jean that we publish a joint paper and he accepted “enthusiastically” (his word). I still have most of the correspondence from that point on. Very unfortunately, I cannot find his first letter about the common pattern of the two systems. It probably was misplaced or taken by someone in the lab. We briefly reported our findings in Microbial Genetics Bulletin #6, which appeared in April 1952 (I enclose copies). We agreed to do some more experiments to complete the comparison P2/lambda, and to write or finalize the joint paper together in Pasadena in March 1952. That is when Jean had his second heart attack, and my trip had to be postponed to mid May. As a consequence of this delay the Luria and Human paper came out ahead of ours, while we were hoping at first that the two papers would appear in the same issue of J.Bact. The discussions with Jean in the preparation of the paper were very interesting because, being a physicist only recently converted to biology, he viewed thing differently from me. Of course all this was before we had any idea of DNA structure and before we had fully digested the implications of the Hershey & Chase experiment. Besides, there was no certainty at the time that P2 and lambda would resemble the T phages in composition.
Looking back at the period when the first examples of restriction and modification were observed up to the first attempts at biochemical analysis, I'm struck by the following: (1) There were several examples of the phenomenon cropping up (for example the one on Staphylococcus phage by Ralston & Krueger, Proc. Soc. Exp. Biol. Med. 80:217, that was published as we were working on our manuscripts) and only a few happened to be seen as seriously problematic at the time and investigated further. [On reviewing my old protocols I discovered another example of this in my own work of February 1950 (!), a case which I had completely forgotten and failed to pursue at the time.] (2) The immediate reaction was to think in terms of known non-genetic effects, like “phenotypic mixing” or the heterogeneity of phage particles in respect to heat stability. (3) The realization that the distinction genotype/phenotype was breaking down (i.e. that one had reached the limits of standard formal genetics) came slowly. When Jean and I were working on our joint paper, we had several discussions on the meaning of “genetic”. The question is also discussed at some length in Luria's Cold Spring Harbor paper (C.S.H.Symp. 18:237; 1953).
As intriguing as host modification was, I realized that I would not be able to continue on both it and lysogeny as my main research activities, so that I became a spectator as far as host controlled variation was concerned. An exception was when Allan Campbell got a bright idea for testing whether DNA was involved in the effect: he spent the summer of 1956 with us and we did together a few (very complicated) experiments with P2 in B. The experiments did not work well enough, though, and we abandoned them. Also, much later, in Stockholm, I believe in the summer of 1964, I organized a small informal meeting on nucleic acid methylation, which was attended by Arber, Campbell, Seymour Lederberg and a few others.
Jean Weigle came to Caltech in 1949, but he used to spend summers in Geneva in the biophysics lab that was part of his former physics department, so that he had quite a bit of influence on the direction of the work there and was for many years a direct connection between Geneva and Caltech. Werner Arber finished his doctorate in 1958 at Geneva working on Gal transduction by lambda, then spent a year in our lab at the University of Southern California, working on transduction by P1 of lambda prophages and of the F factor (Virology 11:250 & 11:273).
Sorry for taking so long to put together this letter.
With kind regards,
APPENDIX 2: BERTANI OBITUARY2
Giuseppe Bertani
Professor Giuseppe Bertani (Joe to friends) died on April 7, 2015 at the age of 91 in Pasadena, CA. As a pioneering microbial geneticist, his insights helped to develop both modern microbiology and the molecular biology of today. Born in Como, Italy, Joe was raised in Milan, where he earned his doctorate in zoology. After postgraduate studies in Naples and Zürich, he arrived at the Cold Spring Harbor Laboratory (CSHL) in October 1948 as a Carnegie Fellow working in Milislav Demerec's group. Here, he shifted his focus to bacterial genetics and was soon measuring reverse mutation rates in a streptomycin-dependent mutant strain of Escherichia coli after exposure to radiation and chemical agents; in fact, these experiments preceded what would later become the Ames test. Most importantly, it was here that Joe was shown phage plaques for the first time by his friend Gus Doermann, who was working on phage T4, and that he first encountered lysogeny.
Joe attended Max Delbrück's phage course at CSHL in 1949, after which he joined Salvador Luria at Indiana University in Bloomington. Here he began studying lysogeny, although at first Luria was somewhat reluctant. Using what he called a “modified single burst technique” Joe demonstrated that phage production by a lysogen was discontinuous, involving rare, large bursts of phage. He went on to characterize the establishment of lysogeny, the state of the prophage, and superinfection immunity. As it turned out, the Lisbonne strain Joe was using produced three different phages, which he named P1, P2, and P3. It was P2, the noninducible phage, which was to become his primary phage of study. During these studies Joe composed the now ubiquitous LB medium, which subsequently has been referred to as Luria broth, Lennox broth, or Luria-Bertani medium. For the historical record, Joe pointed out that the abbreviation LB was intended to stand for “lysogeny broth.” In addition to his lysogeny work, Joe's discovery in 1953 of “host-controlled variation,” together with Jean Weigle, ushered in our understanding of host restriction and modification, which influenced the discovery of restriction enzymes 15 years later.
Joe remained with Luria after the lab moved to the University of Illinois in 1950, where he met and married Betty, and then in 1954 Joe joined the laboratory of Max Delbrück at Caltech. In 1957 Joe took up a professorship in the medical school at University of Southern California in Los Angeles, where Werner Arber joined him as a research associate from 1958–59.
In the early 1960s Joe was appointed professor in microbial genetics at the Karolinska Institute and studies of phage P2 became the focus of the Bertani lab. In these years a steady stream of postdoctoral fellows filled his laboratory in addition to his students and many distinguished visitors. In addition to his obligations at the Karolinska Institute he was also responsible for the advanced teaching of microbiology at the University of Stockholm. His influence on the scientific community in Sweden was significant and his work was recognized by Uppsala University where he received an honorary doctorate in 1982. During this time he also participated in establishing the European Molecular Biology Organisation (EMBO). In 1981 he returned to California to take up a position at the Jet Propulsion Laboratory (JPL) in Pasadena, where he studied the genetics of methanogenic bacteria and described a curious phenomenon of transduction. After formally retiring from JPL in 1991, Joe continued as a voluntary scientist in the Division of Biology at Caltech.
Joe was highly critical but generous when it came to publishing. He rarely put his name on his students work when they were ready to publish their results. Joe Bertani was an outstanding scientist with a philosophical touch, belonging to that dwindling group of pioneers in microbial genetics with roots in the legendary Phage Group. We thank him for taking us on a marvelous journey in science, with him as our guide, and for his friendship. Our thoughts are with his wife Betty, their sons Christofer and Niklas, and their families.
Richard Calendar
University of California, Berkeley
Elisabeth Haggård-Ljungqvist
Stockholm University
Björn H. Lindqvist
University of Oslo
Steven E. Finkel
University of Southern California
1See Appendix 1 (letter) and Appendix 2 (Joe Bertani's obituary).
2Reprinted, with permission, from the American Society for Microbiology (Microbe, January 2006, pp. 20–24).

